1Department of Pediatric Surgery, Seoul National University Children's Hospital, Seoul, Korea.
2Department of Pediatric Surgery, Seoul National University College of Medicine, Seoul, Korea.
3Department of Surgery, Chung-Ang University College of Medicine, Seoul, Korea.
Copyright © 2017 Korean Association of Pediatric Surgeons
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
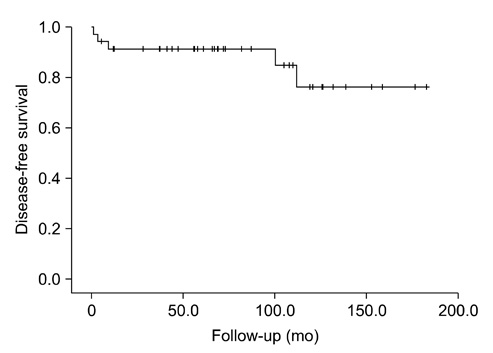
Values are presented as mean±SD or n (%).
LN, lymph node; FNA, fine needle aspiration.
Values are presented as mean±SD or n (%).
CLND, central lymph node dissection; LN, lymph node.
Values are presented as n (%) or mean±SD.
LN, lymph node.
Values are presented as n (%), median (range), or mean±SD.
RAI, radioactive iodine; LN, lymph node; MRND, modified radical neck dissection.
a)Fivepatients underwent lymph node dissection due to recurrence.
b)Period between first operation and reoperation.
F, female; M, male; TT, total thyroidectomy; CND, central node dissection; LND, lateral node dissection; PTC, papillary thyroid cancer; ETE, extrathyroid extension; LN, lymph node; RAI, radioactive iodine.
Values are presented as mean±SD or n (%).
LN, lymph node; FNA, fine needle aspiration.
Values are presented as mean±SD or n (%).
CLND, central lymph node dissection; LN, lymph node.
Values are presented as n (%) or mean±SD.
LN, lymph node.
Values are presented as n (%), median (range), or mean±SD.
RAI, radioactive iodine; LN, lymph node; MRND, modified radical neck dissection.
a)Fivepatients underwent lymph node dissection due to recurrence.
b)Period between first operation and reoperation.
F, female; M, male; TT, total thyroidectomy; CND, central node dissection; LND, lateral node dissection; PTC, papillary thyroid cancer; ETE, extrathyroid extension; LN, lymph node; RAI, radioactive iodine.