Department of Pediatric Surgery, Seoul National University Children's Hospital, Seoul, Korea
∗Department of Pathology, Seoul National University Hospital, Seoul, Korea
Copyright © 2013 Korean Association of Pediatric Surgeons
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
| Antibody | Primary antibody∗ | Company | Dilution | Buffer(pH)† | Target Stain‡ | Cutoff Values§ |
|---|---|---|---|---|---|---|
| Ki-67 | mm | DAKO, Carpinteria, California, USA | 1:200 | CA (6) | N | > 2 % |
| p53 | mm | DAKO | 1:200 | CA (6) | N | > 5 % |
| mdm-2 | mm | Leica Biosystems, Heidelberg, Germany | 1:100 | EDTA (9) | N | > 50 % |
| Santa Cruz | ||||||
| p21 | mm | biotechnology, Dallas, | 1:200 | CA (6) | N | > 10 % |
| Texas, USA | ||||||
| Spring Bioscience, | ||||||
| p27 | rp | Pleasaton, California, | 1:300 | CA (6) | N | > 30 % |
| USA | ||||||
| bcl-2 | mm | DAKO | 1:100 | CA (6) | C | > 50 % |
| cyclin D1 | rp | Santa Cruz biotechnology | 1:100 | CA (6) | N | > 5 % |
| Total (n=20) | PHEO (n=14) | aPGL (n=6) | P value | |
|---|---|---|---|---|
| Age (months, mean±SD) | 143.90 ±40.87 | 141.93 ±39.26 | 148.50 ±19.60 | .775∗ |
| Gender (M : F) | 15 : 5 | 9 : 5 | 6 : 0 | .260† |
| Location | .829† | |||
| Right (%) | 7 (35) | 5 (35.7) | 2 (33.3%) | |
| Left (%) | 6 (30) | 5 (35.7) | 1 (16.7%) | |
| Bilateral (%) | 7 (35) | 4 (28.6) | 3 (50.0%) | |
| Greatest dimension (cm, mean±SD) | 4.86 ±1.50 | 4.84 ±1.26 | 4.88 ±2.08 | .966† |
| Malignancy (%) | 7 (35) | 4 (28.6) | 3 (50.0%) | .613† |
| Hereditary Features (%) | 4 (20) | 4 (28.6) | 0 | .267† |
| Chemotherapy (%) | 2 (10) | 0 | 2 (33.3%) | .079† |
| Radiotherapy (%) | 6 (30) | 3 (21.4) | 3 (50.0%) | .303† |
| Follow-up(months, mean±SD) | 97.25 ±55.67 | 95.64 ±37.03 | 101.00 ±90.47 | .850∗ |
| Disease free survival (months, mean±SD) | 82.20 ±58.25 | 82.43 ±43.86 | 81.67 ±88.82 | .985∗ |
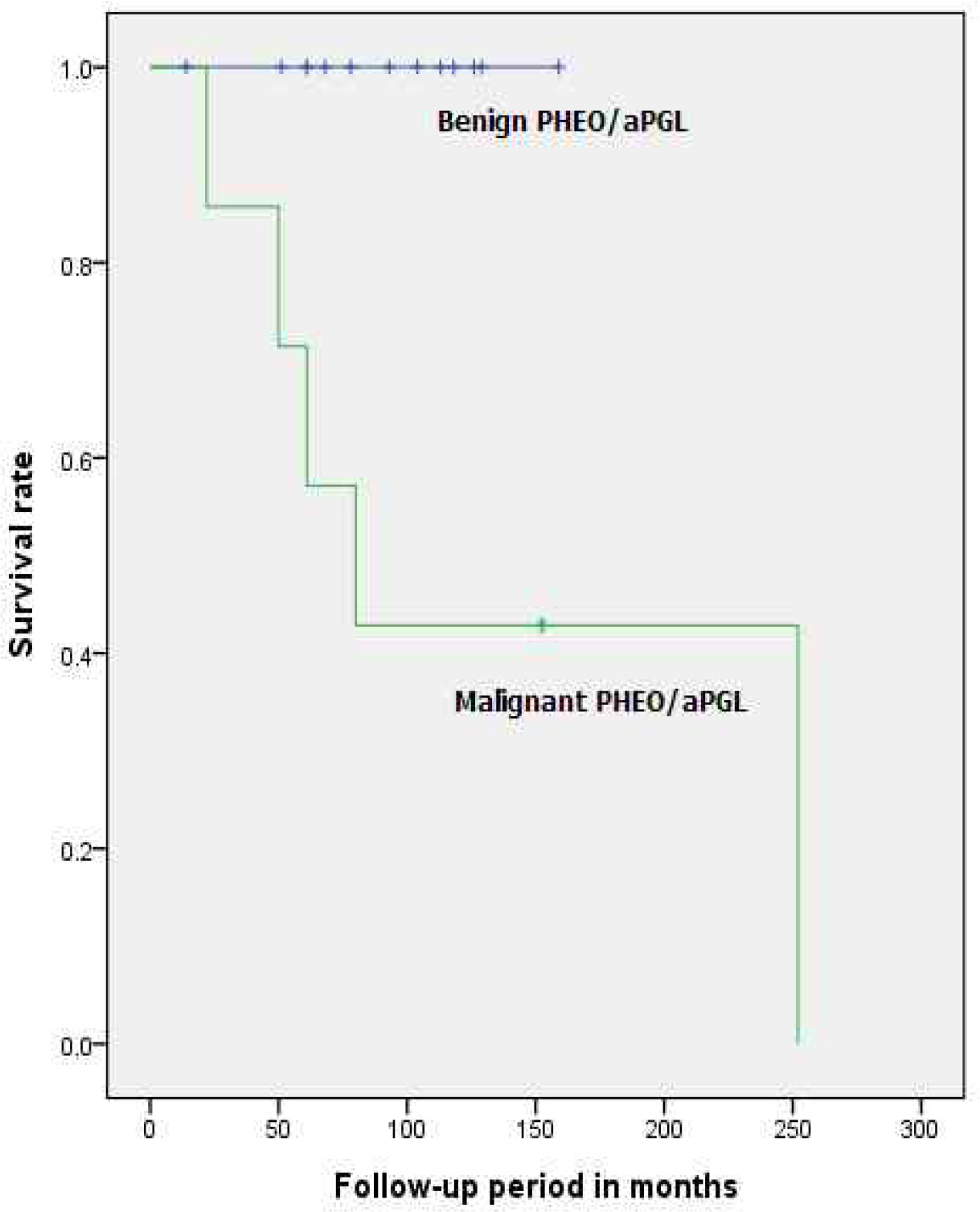
| Median survival (months, range) | 86.20 (14-252) | 86.50 (50-159) | 82.50 (14-252) | NA§ |
| Benign (n=13) | Malignant (n=7) | P value | |
|---|---|---|---|
| Age (months, mean±SD) | 143.77 ± 46.25 | 144.14 ± 31.78 | .983† |
| Gender (M : F) | 10 : 3 | 5 : 2 | > .999‡ |
| Hereditary Features (%) | 3 (23.1) | 1 (14.3) | > .999‡ |
| Chemotherapy (%) | 0 | 2 (28.6) | .111‡ |
| Radiotherapy (%) | 0 | 6 (85.7) | < .001‡ |
| Location | .381‡ | ||
| Right (%) | 5 (38.5) | 2 (28.6) | |
| Left (%) | 5 (38.5) | 1 (14.3) | |
| Bilateral (%) | 3 (23.1) | 4 (57.1) | |
| Greatest dimension (cm, mean±SD) | 5.01 ± 1.55 | 4.57 ± 1.44 | .547† |
| Clinical manifestation | |||
| Hypertension (%) | 10 (76.9) | 4 (57.1) | .613‡ |
| Palpitation (%) | 7 (53.8) | 3 (42.9) | > .999‡ |
| Headache (%) | 6 (46.2) | 2 (28.6) | .642‡ |
| Diaphoresis (%) | 7 (53.8) | 2 (28.6) | .374‡ |
| Flushing (%) | 9 (69.2) | 2 (28.6) | .160‡ |
| Nausea, vomiting (%) | 7 (53.8) | 3 (42.9) | > .999‡ |
| Follow-up (months, mean±SD) | 90.38 ± 39.48 | 110.00 ± 80.02 | .467† |
| Disease free survival (months, mean±SD) | 90.38 ± 39.48 | 67.00 ± 84.92 | .690† |
| Median survival period (months, range) | 93.00 (14-159) | 80.00 (22-252) | NA§ |
| Benign (n=13) | Malignancy (n=7) | P value† | |
|---|---|---|---|
| Capsular invasion (n, %) | 4 (30.8) | 3 (42.9) | .651 |
| Vascular invasion (n, %) | 0 | 4 (57.1) | .007 |
| Extension into adipose tissue (n, %) | 1 (7.69) | 4 (57.1) | .031 |
| Presence of large nests (n, %) | 7 (53.8) | 1 (14.3) | .158 |
| Central tumor necrosis (n, %) | 6 (46.2) | 5 (71.4) | >.999 |
| High cellularity (n, %) | 3 (23.1) | 2 (28.6) | >.999 |
| Tumor cell spindling (n, %) | 8 (61.5) | 1 (14.3) | .070 |
| Cellular monotony (n, %) | 3 (23.1) | 1 (14.3) | >.999 |
| Mitosis (n, %) | 1 (7.69) | 4 (57.1) | .031 |
| Atypical mitosis (n, %) | 1 (7.69) | 2 (28.6) | .270 |
| Nuclear pleomorphism (n, %) | 4 (30.8) | 0 | .249 |
| Nuclear hyperchromasia (n, %) | 9 (69.2) | 2 (28.6) | .160 |
| Benign (n=10) | Malignancy (n=4) | P value† | |
|---|---|---|---|
| Capsular invasion (n, %) | 3 (30) | 2 (50) | .580 |
| Vascular invasion (n, %) | 0 | 3 (75) | .033 |
| Extension into adipose tissue (n, %) | 0 | 3 (75) | .003 |
| Presence of large nests (n, %) | 7 (70) | 0 | .023 |
| Central tumor necrosis (n, %) | 5 (50) | 4 (100) | .089 |
| High cellularity (n, %) | 3 (30) | 1 (25) | .857 |
| Tumor cell spindling (n, %) | 7 (70) | 1 (25) | .139 |
| Cellular monotony (n, %) | 3 (30) | 0 | .234 |
| Mitosis (n, %) | 1 (10) | 3 (75) | .019 |
| Atypical mitosis (n, %) | 0 | 1 (25) | .114 |
| Nuclear pleomorphism (n, %) | 3 (30) | 0 | .234 |
| Nuclear hyperchromasia (n, %) | 8 (80) | 0 | .008 |
| Benign (n=13) | Malignancy (n=7) | P value | |
|---|---|---|---|
| PASS Score (mean±SD) | 6.00 ± 2.86 | 7.57 ± 3.36 | .317† |
| PASS ≥ 4 (n, %) | 10 (76.9) | 5 (71.4) | >.999‡ |
| PASS ≥ 6 (n, %) | 8 (61.5) | 5 (71.4) | >.999‡ |
| Pathologic Score (mean±SD) | 0.31 ± 0.86 | 3.43 ± 2.57 | .001† |
| Score < 2 (n, %) | 12 (92.3) | 2 (28.5) | .007§ |
| Score ≥ 2 (n, %) | 1 (7.7) | 5 (71.4) |
| Antibody | Benign (n=13) | Malignancy (n=7) | P value† |
|---|---|---|---|
| Ki-67 (n, %) | 2 (15.4) | 4 (57.1) | .122 |
| p53 (n, %) | 2 (15.4) | 3 (42.9) | .290 |
| bcl-2 (n, %) | 8 (61.5) | 4 (57.1) | >.999 |
| mdm-2 (n, %) | 2 (15.4) | 3 (42.9) | .290 |
| cyclin D1 (n, %) | 0 | 0 | >.999 |
| p21 (n, %) | 3 (23.1) | 3 (42.9) | .613 |
| p27 (n, %) | 2 (15.4) | 3 (42.9) | .290 |
Immunohistochemical Panel
| Antibody | Primary antibody |
Company | Dilution | Buffer(pH) |
Target Stain |
Cutoff Values |
|---|---|---|---|---|---|---|
| Ki-67 | mm | DAKO, Carpinteria, California, USA | 1:200 | CA (6) | N | > 2 % |
| p53 | mm | DAKO | 1:200 | CA (6) | N | > 5 % |
| mdm-2 | mm | Leica Biosystems, Heidelberg, Germany | 1:100 | EDTA (9) | N | > 50 % |
| Santa Cruz | ||||||
| p21 | mm | biotechnology, Dallas, | 1:200 | CA (6) | N | > 10 % |
| Texas, USA | ||||||
| Spring Bioscience, | ||||||
| p27 | rp | Pleasaton, California, | 1:300 | CA (6) | N | > 30 % |
| USA | ||||||
| bcl-2 | mm | DAKO | 1:100 | CA (6) | C | > 50 % |
| cyclin D1 | rp | Santa Cruz biotechnology | 1:100 | CA (6) | N | > 5 % |
Clinical Information of the Patients with PHEO and aPGL
| Total (n=20) | PHEO (n=14) | aPGL (n=6) | P value | |
|---|---|---|---|---|
| Age (months, mean±SD) | 143.90 ±40.87 | 141.93 ±39.26 | 148.50 ±19.60 | .775∗ |
| Gender (M : F) | 15 : 5 | 9 : 5 | 6 : 0 | .260 |
| Location | .829 |
|||
| Right (%) | 7 (35) | 5 (35.7) | 2 (33.3%) | |
| Left (%) | 6 (30) | 5 (35.7) | 1 (16.7%) | |
| Bilateral (%) | 7 (35) | 4 (28.6) | 3 (50.0%) | |
| Greatest dimension (cm, mean±SD) | 4.86 ±1.50 | 4.84 ±1.26 | 4.88 ±2.08 | .966 |
| Malignancy (%) | 7 (35) | 4 (28.6) | 3 (50.0%) | .613 |
| Hereditary Features (%) | 4 (20) | 4 (28.6) | 0 | .267 |
| Chemotherapy (%) | 2 (10) | 0 | 2 (33.3%) | .079 |
| Radiotherapy (%) | 6 (30) | 3 (21.4) | 3 (50.0%) | .303 |
| Follow-up(months, mean±SD) | 97.25 ±55.67 | 95.64 ±37.03 | 101.00 ±90.47 | .850 |
| Disease free survival (months, mean±SD) | 82.20 ±58.25 | 82.43 ±43.86 | 81.67 ±88.82 | .985 |
| Median survival (months, range) | 86.20 (14-252) | 86.50 (50-159) | 82.50 (14-252) | NA |
Clinical Information of the Patients with Benign and Malignant Tumors
| Benign (n=13) | Malignant (n=7) | P value | |
|---|---|---|---|
| Age (months, mean±SD) | 143.77 ± 46.25 | 144.14 ± 31.78 | .983 |
| Gender (M : F) | 10 : 3 | 5 : 2 | > .999 |
| Hereditary Features (%) | 3 (23.1) | 1 (14.3) | > .999 |
| Chemotherapy (%) | 0 | 2 (28.6) | .111 |
| Radiotherapy (%) | 0 | 6 (85.7) | < .001 |
| Location | .381 |
||
| Right (%) | 5 (38.5) | 2 (28.6) | |
| Left (%) | 5 (38.5) | 1 (14.3) | |
| Bilateral (%) | 3 (23.1) | 4 (57.1) | |
| Greatest dimension (cm, mean±SD) | 5.01 ± 1.55 | 4.57 ± 1.44 | .547 |
| Clinical manifestation | |||
| Hypertension (%) | 10 (76.9) | 4 (57.1) | .613 |
| Palpitation (%) | 7 (53.8) | 3 (42.9) | > .999 |
| Headache (%) | 6 (46.2) | 2 (28.6) | .642 |
| Diaphoresis (%) | 7 (53.8) | 2 (28.6) | .374 |
| Flushing (%) | 9 (69.2) | 2 (28.6) | .160 |
| Nausea, vomiting (%) | 7 (53.8) | 3 (42.9) | > .999 |
| Follow-up (months, mean±SD) | 90.38 ± 39.48 | 110.00 ± 80.02 | .467 |
| Disease free survival (months, mean±SD) | 90.38 ± 39.48 | 67.00 ± 84.92 | .690 |
| Median survival period (months, range) | 93.00 (14-159) | 80.00 (22-252) | NA |
Microscopic Features of Benign and Malignant Tumors
| Benign (n=13) | Malignancy (n=7) | P value |
|
|---|---|---|---|
| Capsular invasion (n, %) | 4 (30.8) | 3 (42.9) | .651 |
| Vascular invasion (n, %) | 0 | 4 (57.1) | .007 |
| Extension into adipose tissue (n, %) | 1 (7.69) | 4 (57.1) | .031 |
| Presence of large nests (n, %) | 7 (53.8) | 1 (14.3) | .158 |
| Central tumor necrosis (n, %) | 6 (46.2) | 5 (71.4) | >.999 |
| High cellularity (n, %) | 3 (23.1) | 2 (28.6) | >.999 |
| Tumor cell spindling (n, %) | 8 (61.5) | 1 (14.3) | .070 |
| Cellular monotony (n, %) | 3 (23.1) | 1 (14.3) | >.999 |
| Mitosis (n, %) | 1 (7.69) | 4 (57.1) | .031 |
| Atypical mitosis (n, %) | 1 (7.69) | 2 (28.6) | .270 |
| Nuclear pleomorphism (n, %) | 4 (30.8) | 0 | .249 |
| Nuclear hyperchromasia (n, %) | 9 (69.2) | 2 (28.6) | .160 |
Microscopic Features of Benign and Malignant PHEO
| Benign (n=10) | Malignancy (n=4) | P value |
|
|---|---|---|---|
| Capsular invasion (n, %) | 3 (30) | 2 (50) | .580 |
| Vascular invasion (n, %) | 0 | 3 (75) | .033 |
| Extension into adipose tissue (n, %) | 0 | 3 (75) | .003 |
| Presence of large nests (n, %) | 7 (70) | 0 | .023 |
| Central tumor necrosis (n, %) | 5 (50) | 4 (100) | .089 |
| High cellularity (n, %) | 3 (30) | 1 (25) | .857 |
| Tumor cell spindling (n, %) | 7 (70) | 1 (25) | .139 |
| Cellular monotony (n, %) | 3 (30) | 0 | .234 |
| Mitosis (n, %) | 1 (10) | 3 (75) | .019 |
| Atypical mitosis (n, %) | 0 | 1 (25) | .114 |
| Nuclear pleomorphism (n, %) | 3 (30) | 0 | .234 |
| Nuclear hyperchromasia (n, %) | 8 (80) | 0 | .008 |
Analysis of PASS Score and Pathologic Score between Benign and Malignant Tumor
| Benign (n=13) | Malignancy (n=7) | P value | |
|---|---|---|---|
| PASS Score (mean±SD) | 6.00 ± 2.86 | 7.57 ± 3.36 | .317 |
| PASS ≥ 4 (n, %) | 10 (76.9) | 5 (71.4) | >.999 |
| PASS ≥ 6 (n, %) | 8 (61.5) | 5 (71.4) | >.999 |
| Pathologic Score (mean±SD) | 0.31 ± 0.86 | 3.43 ± 2.57 | .001 |
| Score < 2 (n, %) | 12 (92.3) | 2 (28.5) | .007 |
| Score ≥ 2 (n, %) | 1 (7.7) | 5 (71.4) |
Immunohistochemistry
| Antibody | Benign (n=13) | Malignancy (n=7) | P value |
|---|---|---|---|
| Ki-67 (n, %) | 2 (15.4) | 4 (57.1) | .122 |
| p53 (n, %) | 2 (15.4) | 3 (42.9) | .290 |
| bcl-2 (n, %) | 8 (61.5) | 4 (57.1) | >.999 |
| mdm-2 (n, %) | 2 (15.4) | 3 (42.9) | .290 |
| cyclin D1 (n, %) | 0 | 0 | >.999 |
| p21 (n, %) | 3 (23.1) | 3 (42.9) | .613 |
| p27 (n, %) | 2 (15.4) | 3 (42.9) | .290 |
mm, mouse monoclonal; rp, rabbit polyclonal
CA, citric acid; EDTA, Ethylenediaminetetraacetic acid
N, Nuclei stain; C, Cytoplasmic stain
indicates high proliferative group in Ki-67; otherwise indicates group showed over-expression
Student's t-test
Fisher's exact test
Not applicable
Analysis of all patients with PHEO and aPGL
Student's t-test
Fisher's exact test
Not applicable
Analysis of all patients with PHEO and aPGL
Fisher's exact test
Analysis of patients with PHEO only
Fisher's exact test
Analysis of all patients with PHEO and aPGL
Student's t-test
Mann-Whitney test
Fisher's exact test
Number of cases above cutoff values which are listed in Table 1.
Fisher's exact test