1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
2Division of Pediatric Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Copyright © 2015 by the Korean Association of Pediatric Surgeons
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
No potential conflict of interest relevant to this article was reported.
The abstract of this article was presented at the 66th Annual Congress of the Korean Surgical Society, November 28, 2014.
Values are presented as ratio, mean±SD, or n (%).
PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole.
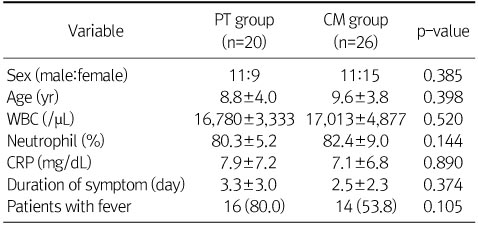
Values are presented as mean±SD, ratio, or n (%).
PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole.
Perioperative Patients Characteristics and Laboratory Findings
Values are presented as ratio, mean±SD, or n (%).
PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole.
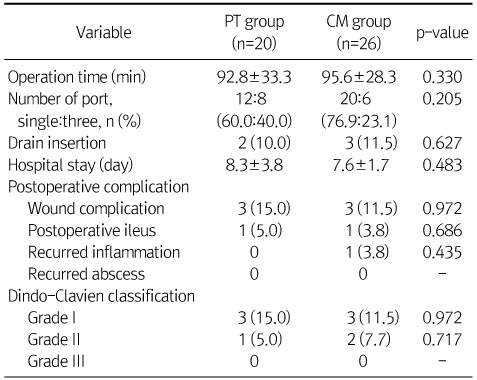
Perioperative and Postoperative Findings
Values are presented as mean±SD, ratio, or n (%).
PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole.
Inflammatory Index according to the Postoperative Day (POD)
Values are presented as mean±SD or n (%).
PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole.
FFS vs. DRG
Values are presented as mean±SD.
FFS, fee for service; DRG, diagnosis related group; PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole; KRW, Korean Won.
Values are presented as ratio, mean±SD, or n (%).
PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole.
Values are presented as mean±SD, ratio, or n (%).
PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole.
Values are presented as mean±SD or n (%).
PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole.
Values are presented as mean±SD.
FFS, fee for service; DRG, diagnosis related group; PT, piperacillin-tazobactam; CM, cefotaxime and metronidazole; KRW, Korean Won.